- 3D Print Bureau
- 6K
- Agmatix
- Airwayz
- AM-Flow
- Appadda
- Caracol
- CG Trader
- CyberOptics
- e-Xstream
- GenCell
- GreenEye
- Impossible Objects
- Incus Media
- InkBit
- ITG
- JPB Systeme
- KeyProd
- Landa
- LEO Lane
- Lumet
- Magic Software
- MakerBot
- Marketiger
- Nano Dimension
- Paragon Rapid Technologies
- PearlX
- Plant & Bean
- Redefine Meat
- Replique
- Ripples
- Sakuu Corporation
- SolarEdge
- StoreDot
- Stratasys
- Sunrock
- The Bespoke Group
- Trigo
- UltiMaker
- Xjet
STRATASYS AND MATERIALISE: 3D PRINTED MEDICAL MODELS COME TO LIFE
Stratasys PolyJet technology and Materialise FDA-cleared software now most versatile 3D printing system for hospitals and physicians to build anatomical models at the point-of-care
Minneapolis & Rehovot, Israel, November 26, 2018 – Further bringing 3D printed medical models to life, Stratasys (Nasdaq: SSYS) is expanding the suite of printers and materials validated by its collaborator Materialise (Nasdaq: MTLS) as part of FDA-cleared Materialise Mimics inPrint software. The end result is the most versatile 3D printing system for point-of-care across hospitals and physicians – advancing production of patient-specific, life-like anatomical models for diagnostic purposes in conjunction with other tools and expert clinical judgement.
PolyJet multi-material and multi-color solutions validated now include the J750 and J735 3D Printers and the high-performance desktop Objet30 Prime 3D Printer. Materialise Mimics inPrint is the first and currently only 3D printing software cleared by the FDA to create anatomical models for patient care. The software is designed to allow physicians and hospitals to leverage 3D printing at point-of-care – building a trusted and reliable source for surgical planning and interdisciplinary communication.
“Historically, pre-surgical planning relied on 2D imaging requiring physicians to mentally reconstruct the patient anatomy. But 3D printing evolves this approach by putting precise replicas of patient anatomy directly in physician hands. Our collaboration with Materialise is a huge step towards unlocking the potential of this technology for patient care,” says Eyal Miller, Head of Healthcare Business Unit, Stratasys. “Now the 3D printer that every hospital needs to power their medical modeling comes with additional options for an FDA-cleared software solution.”
In March of 2018, Materialise Mimics inPrint was cleared by the FDA, becoming the only solution with 510(k) clearance as an end-to-end 3D printing solution, as well as a fully comprehensive 3D printing solution for anatomical modeling. According to company reports, of the top 20 U.S. hospitals as ranked by U.S. News & World Report, 16 have implemented a medical 3D printing strategy using Materialise Mimics technology.
“By validating Stratasys’ 3D printing technologies through our certification process, we’re giving doctors and hospitals improved access to high-quality anatomical models for personalized care to patients,” said Bryan Crutchfield, Vice President and General Manager of Materialise North America. “The addition of multi-color and multi-material printers to the list of validated printers is aimed to enable healthcare providers to implement a versatile offering that can support their most complex cases across a wide range of surgical specialties on a single printer. At Materialise, we take a hardware-agnostic approach to software development, offering the flexibility to partner with other leaders in the 3D printing industry like Stratasys – a company committed to addressing requirements of the medical community.”
The Stratasys J735/J750 3D Printers are able to develop highly-complex models using multiple textures, while combining hard and soft materials to mimic human tissue. The unique combination of transparency with multiple color re-creation ensures practitioners can differentiate anatomy, view critical structures within an organ replica, and create realistic representations of any bone, tissue, and organ.
The Stratasys’ Objet30 Prime 3D Printer is a cost-effective, desktop platform providing an entry point to hospitals seeking a point-of-care printing solution – without compromising quality, resolution or accuracy. The versatile offering supports a range of anatomical models and applications, including orthopedic, cardiac, neurosurgery and other use-cases for visualization, surgical planning, training and education.
For more information on how you can integrate Stratasys’ 3D printing solutions and Materialise software to revolutionize medical modeling practices and improve patient outcomes, visit https://www.stratasys.com/medical
Visit Stratasys at RSNA 2018
Stratasys J750 and Objet30 Prime 3D Printers will be at RSNA 2018 at McCormick Place in Chicago, IL from November 25 to November 30. The company is offering exclusive hands-on demos, detailed customer use-cases and presentations throughout the show at Booth # 1968L, South Hall.
-
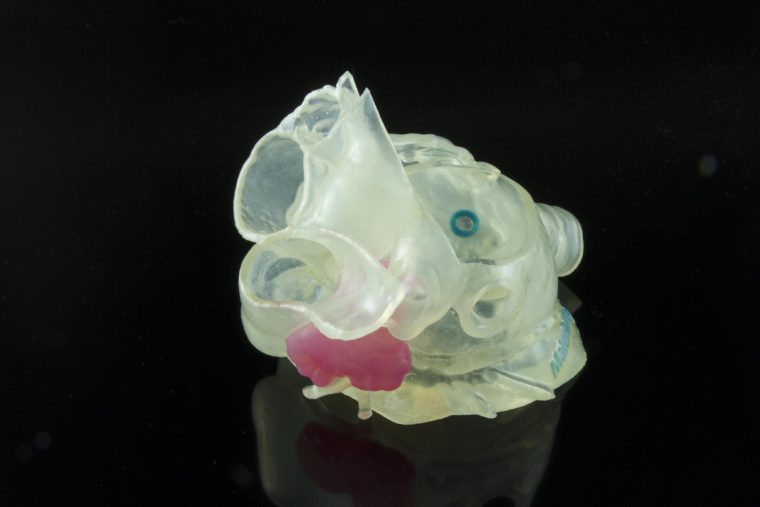 Model of patient’s left atrial appendage (LAA) created with Stratasys and Materialise technology is intended to allow surgeons to select the appropriate device and plan the optimal approach to occlude the LAA. Image provided by Materialise
Model of patient’s left atrial appendage (LAA) created with Stratasys and Materialise technology is intended to allow surgeons to select the appropriate device and plan the optimal approach to occlude the LAA. Image provided by Materialise
Click here to download 300dpi images -
 Creating highly-accurate models using multiple textures that mimic human tissue, the Stratasys J750 3D Printer has eight different materials now validated with the Materialise FDA-cleared software
Creating highly-accurate models using multiple textures that mimic human tissue, the Stratasys J750 3D Printer has eight different materials now validated with the Materialise FDA-cleared software
Click here to download 300dpi images





